SPARTAN uses a two-step process to calculate an electrostatic potential map. The first step is to define a set of points where the electrostatic potential will be calculated. The second step is to calculate the potential at these points and to use colors to map the variation in potential.
Step #1 - Define Points
Many criteria can be used to select points for further study. The most common approach is to find points at which the electron density equals 0.002 au. This is a very low level of electron density and such points are typically found near the outermost fringe of the molecule's electron cloud. Therefore, these points approximately define the size and shape of the molecule.
If we consider ALL of the points where electron density = 0.002 au, we find that they form a shell or three-dimensional surface around the molecule. This surface is called the 0.002 isodensity surface ("isodensity" from "iso" = same and "density" = electron density).
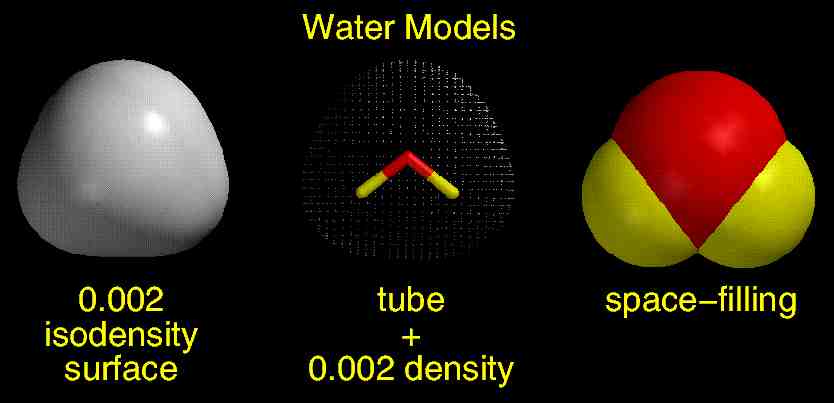
The following figure compares three models of water: a 0.002 isodensity surface, a "tube" model + some of the points where electron density = 0.002 au, and a space-filling model (click on figure for full-size image). The isodensity surface is the collection of ALL points where electron density = 0.002, so this model is identical in shape and size to the collection of points in the middle model. The isodensity surface also has roughly the same shape and size as the space-filling model, and note that both are models are much bigger than the "tube" model (the "tube" model is made by connecting the three atomic nuclei with colored tubes).
Step #2 - Calculate and Map Potential
The electrostatic potential at a point (x, y, z) is given by the electrostatic potential energy between an imaginary positively charged (+1) ion located at (x, y, z) and the molecule. If the ion is attracted to the molecule then the potential is negative. If the ion is repelled by the molecule then the potential is positive. Since the ion has a +1 charge it will be attracted to electron-rich regions of the molecule and repelled by electron-poor regions. Thus, electron-rich regions usually have negative potentials and electron-poor regions usually have positive potentials.
SPARTAN calculates the electrostatic potential at selected points on the 0.002 isodensity surface and maps the surface by color, where different colors are used to identify different potentials (this is like a "rainfall" map where different colors are used to show different amounts of rainfall). The most negative potential is colored RED. The most positive potential is colored BLUE. Intermediate potentials are assigned colors according to the color spectrum:
RED < ORANGE < YELLOW < GREEN < BLUE
Based on this scheme, one can usually identify RED regions of a map as being the most electron-rich regions of a molecule, and BLUE regions of a map as being the most electron-poor regions of a molecule. An electrostatic potential map of a water molecule is shown below, along with a legend that shows how potential varies with color (click on figure for full-size image). Note that the RED region is found near oxygen and the two BLUE regions are found near the two hydrogens. This means that the oxygen is relatively electron-rich in this molecule, and the hydrogens are relatively electron-poor.
Electrostatic Potential Energy
The potential energy of two charged particles depends on the nature of their charges and the distance between them. The energy, E, is given by the following formula:
where Q1 and Q2 are the charges on the two particles and R is the distance between them.
This formula has a simple interpretation. The potential energy is zero when the particles are far apart (large R), but becomes large as the particles approach each other (small R). The energy is especially large if both of the charges are large, but is small if both of the charges are small. Finally, the potential energy is positive (repulsive) when the particles have like charges (Q1 and Q2 both positive, or Q1 and Q2 both negative), but is negative (attractive) when the particles have unlike charges. Some typical relationships between charge, distance, and energy are shown below.
A molecule is a collection of charged particles, negative electrons and positive nuclei, and the potential energy between a molecule and a +1 ion must take all possible electrostatic interactions into account. That is, the potential energy is the sum of the electrostatic interactions between the +1 ion and each of the molecule's nuclei and also the +1 ion and each of the molecule's electrons (the latter are treated collectively by calculating the potential energy between the +1 ion and the electron cloud). Thus, the potential energy is given by the formula:
A useful approximation is to assume that "nearest neighbor" interactions are most responsible for the potential energy. That is, we focus our attention on the nuclei and electrons closest to the +1 ion. If the ion is near an electron-rich atom (electron charge greater than nuclear charge) the potential energy will usually be negative. On the other hand, if the ion is near an electron-poor atom (nuclear charge is greater than electron charge) the potential energy will usually be positive.